Sanavo: Ending the Death Sentence of Glioblastoma
Here is an image of a normal, healthy brain. Pay close attention to the white structure in the middle:

And here are examples of two similar patients with glioblastoma. Notice how those two blue lines (representing the cerebral ventricles), which should normally be symmetrical along the middle of the brain, have been either pushed to the side or compressed by the red outline, representative of a glioblastoma tumor.
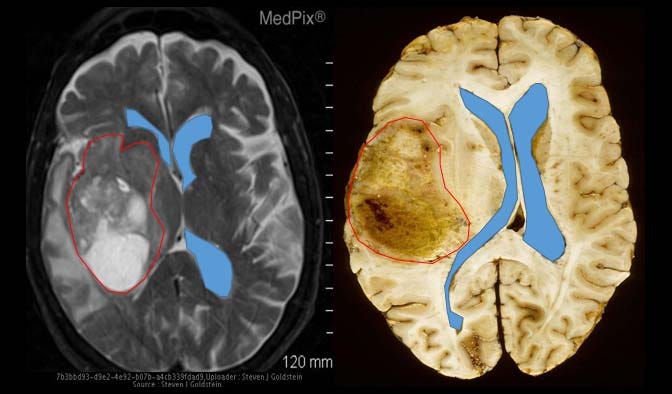
Imagine that you are a patient and you just received an MRI scan showing similar results as above.
You’ve just received a death sentence: a glioblastoma tumor diagnosis.
Current Treatment Methods
Glioblastoma is the most common malign cancer that affects the brain and central nervous system; it accounts for around one in six brain tumors. Glioblastoma is considered a grade 4 glioma brain tumor, arising from glial cells, specifically a sub-category of glial cells called astrocyte cells (a brain tumor’s grade refers to how likely the tumor is to grow and spread, grade 4 is the most aggressive and serious type).
The tumor’s cells are abnormal, and the tumor creates new blood vessels as it grows. Given your results, you are now lined up with a three-step treatment plan: surgery (craniotomy), chemotherapy, and radiation therapy.
Currently, the first treatment step — surgery — is determined by the results of the MRI scan. Most patients with glioblastoma first have a CT scan of the brain when they go to the doctor. If a tumor is found and there is no bleeding, an MRI with standard T2-weighted (T2w), T2-fluid-attenuated inversion recovery (T2-FLAIR), gradient echo, T1-weighted (T1w), and T1-weighted contrast-enhanced (T1CE) sequences. Sequences, in this context, refers to the different types of images produced during an MRI. Each sequence uses specific MRI settings to highlight various features and properties of the brain tissue. Neurosurgeons use utilize high-resolution MRI (0.5–1.2 mm slice thickness) to help plan and guide the surgery and decide how much of the tumor can be safely removed without harming important areas of the brain.
Advanced MRI techniques like functional MRI (fMRI) are crucial before surgery, especially when the tumor is near critical brain areas. This helps plan a safer and more effective surgery. Other techniques like diffusion tensor imaging (DTI) also produce detailed images of brain pathways, aiding in surgical planning, and distinguishing between effects of surgery and remaining tumor tissue.
After your surgery, it’s common to use an MRI scan within 24–48 hours to better assess how much of the tumor was removed. Usually around 97% of the cancer cells will be removed. While 97% is a lot, you need to remove every single cancer cell to kill the glioblastoma tumor. If any cancer cell remains, the tumor will grow back. Furthermore, research has shown that removing a brain tumor causes remaining cancer cells to grow 75% faster than the original tumor, meaning that the cancer will multiply quickly and spread to other areas in spite of the surgery. Furthermore, potential side and long-term effects after brain surgery include swelling (oedema) in the brain, difficulty walking, weakness in an arm or leg, difficulty concentrating or remembering things, problems with speech, fatigue, and epilepsy.
After surgery, the next part of your treatment plan will involve chemotherapy and radiation therapy to remove all remaining cancer cells. This treatment usually starts 3 to 6 weeks after surgery, giving the patient time to recover. When planning the radiotherapy, doctors use the MRI taken after surgery along with a CT scan to carefully plan the treatment area, focusing on any remaining tumor and affected brain tissue. In radiation therapy, powerful energy beams from X-rays and protons kill cancer cells. Radiation is often combined with chemotherapy, where strong drugs (typically temozolomide, or TMZ) are used to kill cancer cells.
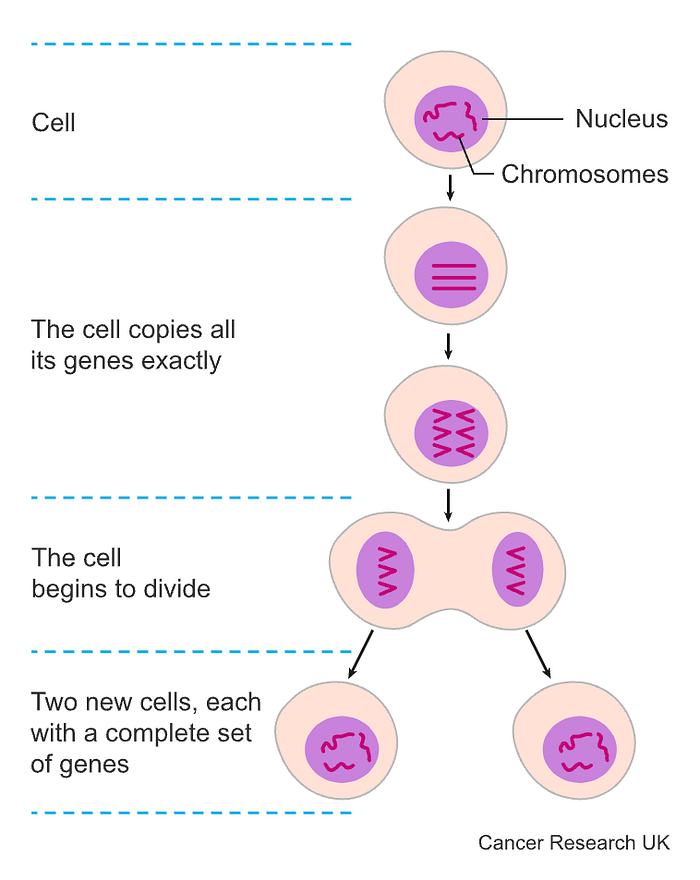
Chemotherapy
Chemotherapy works by killing any cell that is replicating fast. In cancer, the cells divide super quickly until there is a huge mass of cells, which become a lump, or tumor. Because cancer cells divide much faster than most regular cells, chemotherapy is much more likely to kill them than other cells.
In each living cell is a nucleus, the cell’s control center. The nucleus contains chromosomes, which consist of genes and are the target of chemotherapy drugs. Some drugs may damage the cells at the point of splitting while others damage the cells before they split — while the cells are replicating their genes. You might also have a blend of different chemotherapy drugs which damage cells at different stages of cell division, increasing the chance of killing cancer cells.
Your chemotherapy can be administered in a variety of ways:
1) Intravenous chemotherapy
In this method, you can have treatment through a thin short tube (a cannula) that enters a vein in your arm each treatment session, or you might have treatment through a tunnelled central line. Long plastic tubes inject the drug into a large vein in your chest. A huge portion of the line remains in your body for months to be used to either extract blood or inject more treatment.
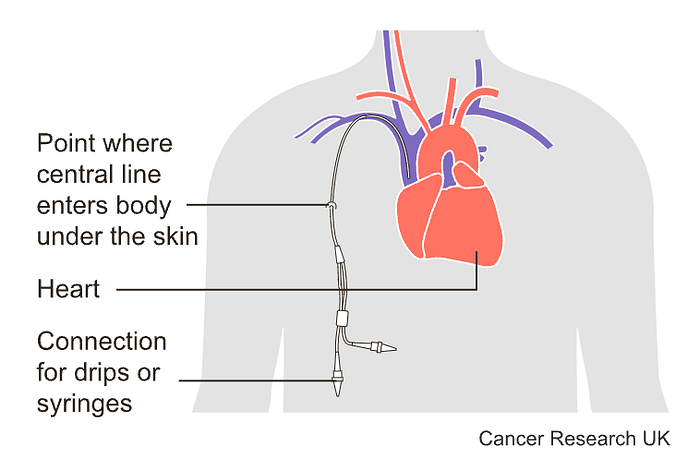
Sometimes problems may occur with tunneled central lines:
- You may get an infection.
- The line may get blocked.
- A blood clot can develop.
- The line may split, although rarely.
The main problem with injecting chemotherapy drugs into the blood is that they can affect healthy body tissues where the cells are constantly growing and dividing, such as:
- Your hair, which is always growing
- Your bone marrow, which is constantly producing blood cells
- Your skin and the lining of your digestive system, which are constantly renewing themselves
2) Intrathecal chemotherapy
This treatment involves injecting chemotherapy drugs into the fluid-filled space between the thin layers of tissue that cover the spinal cord and brain.
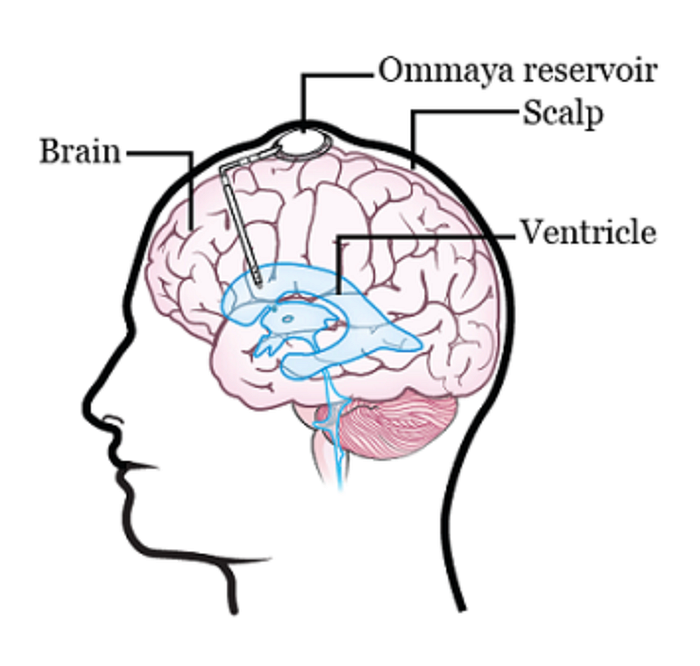
There are two different ways to do this. One way is to inject the drugs into an Ommaya reservoir (a dome-shaped container that is placed under the scalp during surgery; it holds the drugs as they flow through a small tube into the brain). The other way is to inject the drugs directly into the cerebrospinal fluid (CSF) in the lower part of the spine.
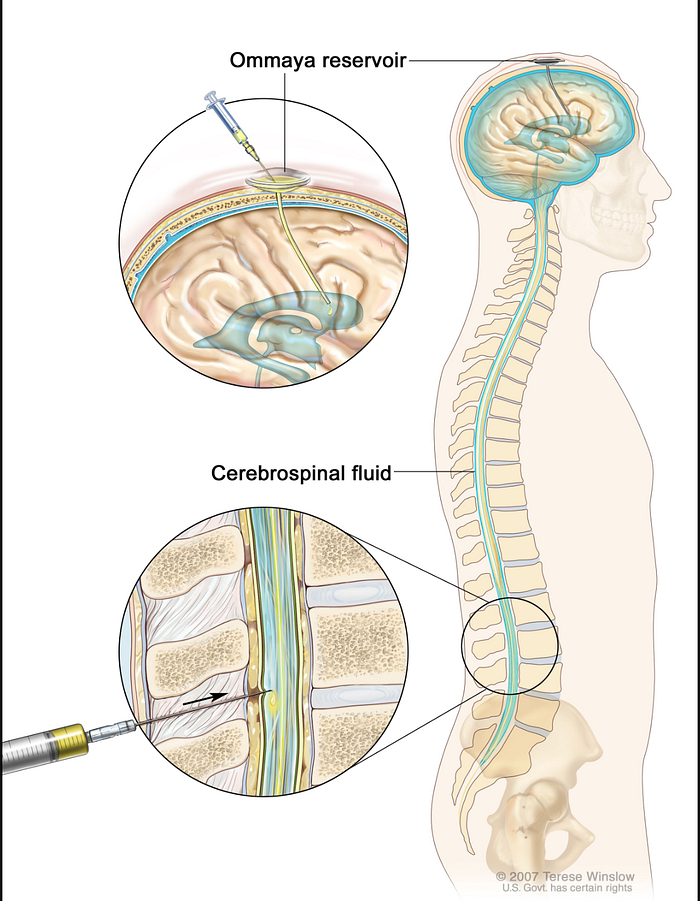
The main issue with the Ommaya reservoir is that surgery is required to both implant and remove the reservoir itself, raising the likelihood of getting an infection or other side effects. Furthermore, you are limited in your activities as you can’t do anything that would risk damaging the reservoir (e.g. contact sports) and you must be constantly wary about hitting your head. Here is a breakdown of all of the risks involved with this procedure:
- There is a small risk that you could bleed into your brain.
- There is a small risk that you could have some loss of function.
- There is a small risk that you could get an infection in your brain.
- The Ommaya reservoir may need to be adjusted. To make sure it’s in the right place, you will get a computed tomography (CT) scan the day after your surgery. If your reservoir isn’t in the right place, you may need to undergo another surgery to correct it.
- The Ommaya reservoir may fail. To make sure your Ommaya reservoir is working, a CSF flow study may be done after your surgery.
3) Chemotherapy wafers
In this method, a surgeon will put chemotherapy drugs into the brain tissue as a wafer (e.g. Gliadel wafer). The chemotherapy drugs are inside the gel wafer and as the wafer slowly dissolves over 2 to 3 weeks the chemotherapy is also slowly released into the brain tissue.
However, the rigid structure of gliadel wafers limits drug loading capacity, and they might be dislodged from the original site of implantation, missing the target tumor. Furthermore, gliadel wafers require surgery to be implanted. Wafers have also led to the occurrence of seizures, intracranial hypertension, meningitis, cerebral edema, and impaired wound healing in neurosurgical patients
4) Oral chemotherapy
Oral chemotherapy allows you to swallow a tablet that will dissolve under the tongue. This removes the need for needles, IV lines, and constant trips to the hospital.
However, this process is strict and the patient must follow set rules to ensure effective treatment. It is critical to take oral chemotherapy drugs according to the exact schedule that the doctor recommends. The medication may be less effective if a person misses a dose, takes two doses too close together, or takes other drugs alongside the chemo.
Strict rules on dosing mean that you must remember to order new prescriptions on time, which sometimes requires ordering prescriptions days or weeks in advance. People who do not keep up with the ordering process may miss doses, which may ruin their treatment.
In addition, you must take special precautions (such as wearing gloves) when handling the chemotherapy tablets. The medications also require specific storage:
- The tablets must be kept in their original container
- The tablets must be away from other medications
- In a cool, dry place away from heat, sunlight, or moisture
While oral chemotherapy does provide convenience, all of these strict rules mean that the patient has to be constantly on top of their treatment, which can be annoying and tiresome.
Oral chemotherapy also has the same effects as intravenous chemotherapy: it will also kill healthy cells that are dividing. In addition, this oral chemotherapy is expensive, costing around $180–2,600 per month. Given that oral chemotherapy is usually for around 3–6 months, these costs can add up.
Given these options, chemotherapy treatment seems to be a mixed bag of poor solutions. All have their own complexities and complications that are detrimental.
The Problems with Glioblastoma Treatment
Radiation therapy is usually done for 5–8 weeks, 5 days a week. Chemotherapy is usually done alongside radiation therapy, but chemotherapy is done every day. Four weeks after the end of radiation therapy, you may enter the monotherapy phase of treatment. This involves taking TMZ for the first five days of a 28-day cycle.
You can also face side effects from radiation and chemotherapy, including fatigue, hair loss, easy bruising and bleeding, infection, anaemia (low red blood cell counts), nausea, and kidney problems.
After all of this, your treatment plan comes at a steep cost. The average cost of glioblastoma treatment, depending on how long your treatment is, comes to $160,974 for 6 months post-diagnosis, $201,749 for 12 months post-diagnosis, and $268,031 for up to 5 years post-diagnosis.
Alright, but maybe this price is worth it if it ensures you survive. After all, your life is priceless. Unfortunately, the most devastating part about glioblastoma is its survival rate. The average glioblastoma survival time with treatment is 14–16 months from diagnosis. Only 25% of patients survive more than one year, and only 5–7% survive more than five years.
Every year, around 250,000 people are diagnosed with glioblastoma and are forced to go through this process. On top of that, about 200,000 people die each year from glioblastoma as the tumor grows and destroys essential tissue in your brain.
So why is glioblastoma so deadly, and why can’t we treat it properly? As with all cancers, glioblastoma is caused by DNA mutations that result in uncontrolled cell growth. Glioblastoma tumors are especially hard to treat because they aren’t contained in a defined mass with clear borders. The tumor includes thread-like tendrils that extend into nearby areas of the brain. Furthermore, glioblastoma cells have more genetic abnormalities than cells of other types of astrocytoma brain cancer. This genetic diversity within tumor cells often leads to more aggressive behavior, as various mutations enable the cancer to grow rapidly, evade the immune system, resist apoptosis (programmed cell death), and enhance invasiveness. On top of that, the extensive genetic abnormalities make glioblastomas highly resistant to standard treatments like chemotherapy and radiation therapy. Each genetic mutation can potentially alter the tumor’s response to different therapies, making it difficult to eradicate all cancerous cells effectively.
So herein lies the problem: we currently have an extremely expensive treatment that confers an extra 8–11 months of life (you are projected to survive 4 months without treatment). Even worse, approximately 90% of glioblastoma patients will face recurrence within two years of the initial diagnosis. This happens because a few stem-like cells often evade these therapies and reinitiate tumor development. You literally have to eliminate every single cancer cell to prevent tumor recurrence and treat glioblastoma, something that is impossible with current treatments. This means that a patient likely needs checkups every 2–3 weeks to scan the brain and ensure there is no tumor recurrence. These costs can add up as the average cost of a brain MRI is on average around $5,000 (range of $1,600-$8,400). These constant checkups and time being sick lead to an additional indirect cost. One study at a hospital reported that the total indirect cost was $120,131.
To summarize,
- Current treatments are expensive
- Current treatments do not significantly improve the survival rate
- Most glioblastomas recur, even multiple times. This means that patients need to go through the treatment cycle several times and spend much time getting scanned to track tumor recurrence. This can put people in an endless cycle of ineffective treatments.
However, it is important to note that surgery will always play a part in glioblastoma treatment as it is the most effective way to remove a large part of the tumor. The real improvements lie in finding an alternative to chemotherapy and radiation therapy and a treatment that can work when the glioblastoma has gone to healthy tissue and it is hard to preform surgery. Chemotherapy and radiation therapy are incapable of removing all the remaining cancer cells because most of the drug or the delivery vehicle itself doesn’t ever reach the brain. Remember, to treat glioblastoma, you have to remove every single cancer cell, even one remaining one can lead to tumor recurrence. Thus, our treatment solves the last small but critical precent that is the key to truly solving glioblastoma.
So why haven’t we had a new solution? If we can clearly see that our status quo is problematic, what is preventing us from developing a new one?
The Blood Brain Barrier
The blood-brain barrier (BBB) is a network of blood vessels and tissue that helps keep harmful substances from reaching the brain. The BBB is essential for protecting normal brain function by impeding most normal molecules from getting into the brain. No matter what, if you want to get a drug to the brain you must somehow go through the BBB. Although a nasal-to-brain pathway has been explored that can potentially bypass the BBB, several disadvantages make this solution less optimal: low bioavailability (fraction of an administered drug that reaches its target), irreversible damage of nasal mucosa, and a far more limited possible dosage. Pretty much all macromolecules (diameter ranging from about 100 to 10,000 angstroms) cannot penetrate the brain endothelium (tissues that line blood vessels). Furthermore, 98% of small molecules also can’t be transported across the BBB. Only positively charged small lipid-soluble molecules with a molecular weight < 400 Da can naturally cross the BBB. These restrictions make it challenging to design effective therapies that can enter the brain. The BBB consists of tightly packed cells and structures including endothelial cells, pericytes, astrocytes, neurons, and membranes. These components form a strong barrier in brain capillaries. The capillary endothelial cells are tightly joined without gaps, significantly limiting the passage of small molecules and proteins. Additionally, connections between these endothelial cells create a continuous barrier that restricts water-soluble substances from passing through. The permeability of the BBB is largely governed by these connections, including tight junctions, adherent junctions, and gap junctions.
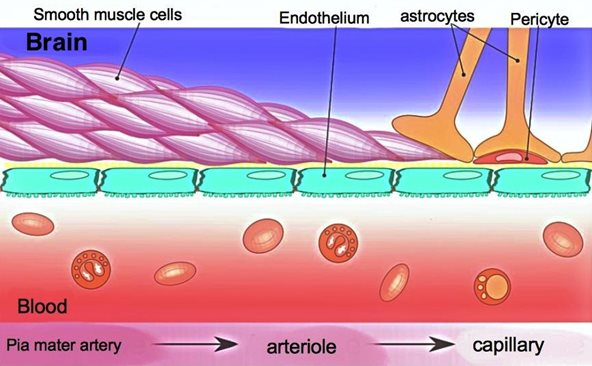
More specifically, adherens junctions mainly control how substances pass through endothelial barriers. Tight junctions are crucial for maintaining the barrier that regulates the passage of materials in epithelial cells and endothelial cells, which helps keep tissues stable. Pericytes, astrocytes, and basal membrane surround the ECs and form the impermeable BBB. Additionally, efflux transporters are located in brain capillary ECs, which are further obstacles against substances entering the brain.
Furthermore, glioblastoma initially mimics the BBB and then eventually damages it as the glioblastoma grows and spreads. Once the glioblastoma is self-sustaining and can survive on its own, it constructs a new barrier called the blood-brain tumor barrier (BBTB). The BBTB has new blood vessels that supply the tumor with nutrients and oxygen and help cancer cells spread to other brain areas. This new barrier is different from the BBB in that it is more permeable (able to be penetrated or passed through). Although the BBTB allows more substances to pass through compared to the BBB, its inconsistent ability to let small and large molecules through and its varied blood flow still cause poor drug delivery to brain tumors. Thus, there is a new challenge to be overcome to create an effective drug delivery system.
Despite these many challenges, our current treatments do not have to remain as the status quo. Glioblastoma does not have to be a death sentence and we can develop a better, cheaper, and less painful solution.
Introducing Sanavo Part 1: Hyaluronic Acid Nanogels
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. It essentially combines the advantages of a hydrogel with nanoparticles. To understand nanogels we must first understand hydrogels. A hydrogel is a three-dimensional network formed by hydrophilic polymers through chemical (covalent or ionic bonds) or physical (hydrogen bonds, van der Waals force, physical entanglement) cross-linking. Hydrogels can swell (enlarge) and retain a significant fraction of water within their structure, but they will not dissolve in water. It is produced by the simple reaction of one or more monomers: a molecule that can be bonded to other identical molecules to form a polymer. Think of hydrogels as essentially a gel: they are stable yet still possess a fluid and free-flowing quality.
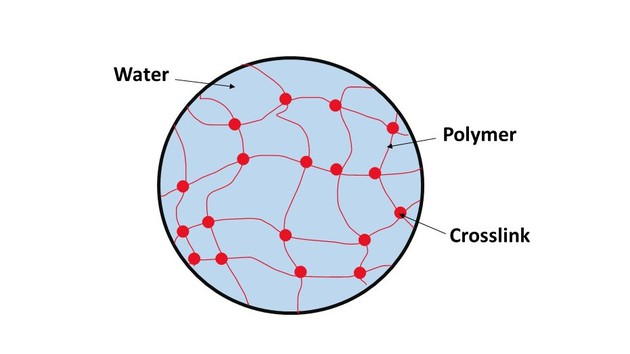
The ability of hydrogels to absorb water arises from hydrophilic (tending to interact with water) functional groups attached to its backbone, while their resistance to dissolution arises from cross-links between network chains. Hydrogels are also stable in conditions of sharp and strong fluctuations in temperature.
Along with those advantages, natural polymers are ideal skeletons for hydrogels because of their diversified properties such as their biocompatibility, biodegradability, and environmental friendliness. Natural polymers can be defined as polymers that are formed from photosynthesis, biochemical reactions in the natural world, or extracted from natural products. Hydrogels based on natural polymers (eg., alginate, starch, cellulose, chitosan, gelatin, collagen, hyaluronic acid) show good degradability, biocompatibility, nontoxic degradation products, good flexibility similar to natural tissue, and are in natural abundance.
Biocompatibility is one of the most significant characteristics of hydrogels for biomedical applications, referring to the ability of a material to connect with bodily organs with minimum damage to the surrounding tissues and without triggering undesirable immune responses. Hydrogels based on natural polymers usually have excellent biocompatibility due to the intrinsic properties of natural polymers.
Biodegradability, the capacity of a substance to break down after interactions with biological elements, is another essential property of hydrogel materials for biomedical applications. Most hydrogels made from natural polymers can be broken down by enzymes. The speed at which these hydrogels decompose depends on factors such as the polymer’s molecular weight and whether the polymer is more amorphous (lacking a clearly defined shape) or crystalline (having the structure/form of a crystal), and if it is hydrophilic or hydrophobic.
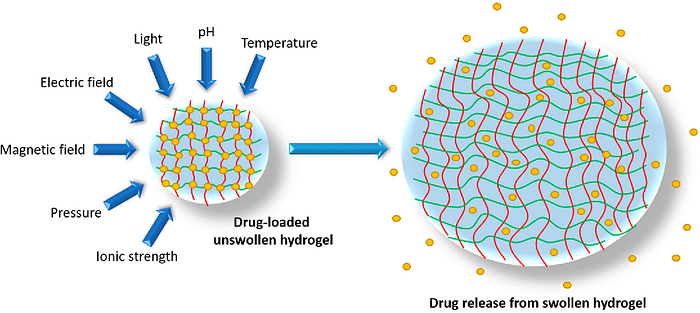
Now that we understand the importance of hydrogels, lets return to nanogels. A nanogels characteristics, such as size, charge, porosity, amphiphilicity, softness, and degradability, can be fine-tuned by varying their chemical composition. They can be designed in a spherical shape or in a more porous structure, with holes in their shape. From a drug delivery perspective, this essentially allows us to design the system based on how we want the drug to be released. Based on the structure, drugs can be released in different orders. For example, in a spherical shape, the outermost contained drug would be released first; however, in a porous structure, you could theoretically have the innermost structure released first. The porous structure is sort of like Swiss cheese in that there are ways to get to the middle of the structure, allowing you to theoretically release drugs from the inside out.
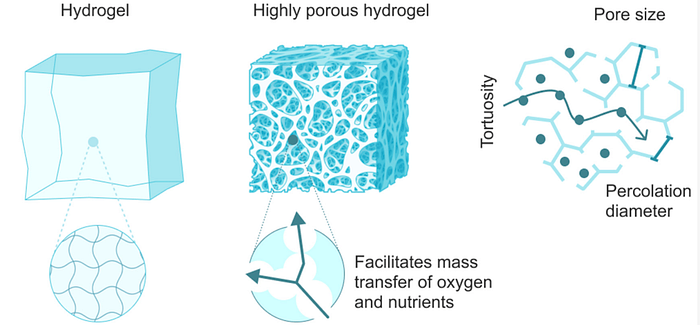
Here is a full list of the advantages of nanogels:
- Being mostly hydrophilic in nature, nanogels are highly biocompatible with a high loading capacity for guest molecules. This is important because we want the drug delivery system to be able to hold a high amount of cargo so that it can properly attack the entire tumor and not run the risk of running out of cargo. As previously mentioned biocompatibility is important to ensure that we do not illicit an immune response.
- Nanogels not only protect the cargo from degradation and elimination but also participate actively in the delivery process due to their characteristic properties, like stimuli-responsive behaviour (responding to changes in the body) softness and swelling to help achieve a controlled, triggered response at the target site. Protecting the cargo is important to ensure the drug isn’t accidentally released in the wrong place. Softness is also critical to ensure the drug delivery system does not scratch or harm internal organs. Finally, as will be discussed later, this stimuli-responsive behavior is key as the hydrogel can essentially be programmed to release the drug based on certain conditions in the body.
- The versatility of their architecture allows for the incorporation of a range of molecules, from inorganic nanoparticles to biomacromolecules like proteins and DNA. Both hydrophilic and hydrophobic drugs can be formulated in nanogels. This is extremely important because as we develop newer and more effective therapies, we will want this system to remain useful and still applicable.
- Nanogels prevent biomolecules like enzymes and genetic material from degradation while their macromolecular properties help increase the circulation half-lives of small molecules.
- Nanogels serve as a highly convenient platform for combination delivery of therapeutic molecules. Combination drug delivery refers to the approach of using two or more therapeutic agents delivered together using a single delivery system. Combining drugs can often lead to enhanced therapeutic effects as the drugs can work synergistically to treat disease more effectively than either drug could alone. It also requires less dosage as each drug can be used at a lower dose than if used alone, potentially reducing the side effects associated with higher doses of single drugs.
- They can be targeted specifically to the site of interest by conjugation (bonding) with a targeting ligand or due to the passive targeting that is a characteristic feature of their nanoscale size.
- Their very rapid response to a change in environmental conditions. They can adapt to different pH, temperature, and other characteristics in the body, which is important given how varying the climate is and we can’t control what happens in vivo. The responsiveness of nanogels to the external physical or chemical signals can also be tightly regulated by controlling the structure of the materials used for the preparation of the nanogels.
- Nanogels are highly swollen and can incorporate 30% of their weight in biological molecules and drugs. These loading capacities are unusually high and exceed those of liposomes and polymeric micelles.
- Nanogels are quite small (30–50 nm) and flexible, meaning that they have a great chance of crossing the BBB.
Hyaluronic Acid (HA)
Hyaluronic acid is a natural polysaccharide found in the extracellular matrix prominently throughout the body. It is comprised of N-acetyl-glucosamine and D-glucuronic acid residues. The extracellular matrix (ECM) helps cells attach to, and communicate with, nearby cells, and plays an important role in cell growth, cell movement, and other cell functions. As a main component of the ECM, hyaluronic acid plays a significant role in lubrication, water absorption, and retention for tissue and the ECM, structural and space-filling functions, and interacts with various cell receptors to coordinate cell communication and behavior.
So why did we choose our nanogel to be made out of hyaluronic acid? We choose hyaluronic acid because it is well known for its bioactivity, which means that it can produce a certain therapeutic effect when interacting with biological systems. Hyaluronic acid also has other advantageous properties such as its nontoxicity, nonallergy, biocompatibility, and biodegradability.
The chemical modification of hyaluronic acid is mainly focused on three distinct functional groups: the glucuronic carboxylic acids, the primary and secondary hydroxyl groups, and the N-acetyl groups. Altering any of these functional groups can result in changes in the properties (mechanical or chemical) and biological activity of hyaluronic acid. This is important because we want our system to be customizable to fit the needs of a specific patient based on where the tumor is located or what specific drug we are using.
Hyaluronic acid hydrogels can be formed by auto-cross-linking within a single molecule and between different molecules. This happens through a reaction between the carboxyl and hydroxyl groups, leading to the formation of ester bonds, which increases the hydrogels’ viscoelasticity (how it stretches and recovers) Finally, by adjusting the conditions under which this bonding reaction occurs, we can control the stiffness and stretchiness of the hydrogels.
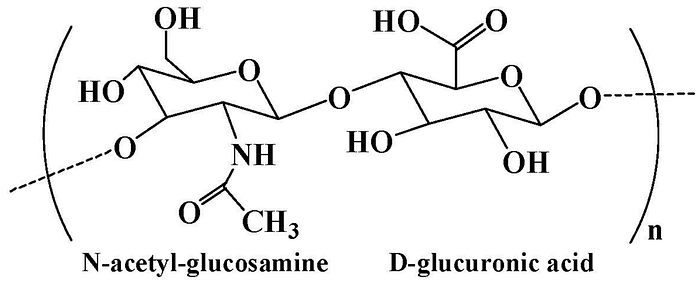
Hyaluronic acid contains hydroxyl and carboxyl functional groups in the main chain, which can be chemically modified to obtain hyaluronic acid derivatives with unique biological and physicochemical properties. The carboxyl group in hyaluronic acid can be changed to create derivatives. Ester derivatives can be made using alkyl halides, diazomethane, or tosylate activation and amide derivatives can be formed using carbodiimides or carbonyldiimidazole. Furthermore, hyaluronidase, an enzyme that breaks down hyaluronic acid, is found at higher levels in many cancer cells. Because of this, hyaluronic acid-based hydrogels, which can be broken down by the body, are often used to deliver local therapies. While I am talking about hydrogels here, it is important to note that these characteristics can still be applied to nanogels, which are just a smaller version of hydrogels.
Hyaluronic acid is primarily produced either by extracting it from animal tissues, such as human umbilical cords, the vitreous humor of cattle, bovine synovial fluid, and rooster combs, or through microbial fermentation using both pathogenic (causing disease) and nonpathogenic bacteria. It is significant that hyaluronic acid can be found in our bodies or animals as it drastically reduces costs as opposed to chemically producing this polymer. Hyaluronic acid is often used in labs already and the average retail price of it is typically around $59.69 per 3, 30 capsules bottle.
A full list of the most important advantages of hyaluronic acid:
- Viscoelasticity (viscous = thick and sticky, elastic = stretchy)
- Biocompatibility
- Biodegradability
- High water absorption and retention capacity, make it a versatile lubricant, filler, and structural support in the human body.
- Hyaluronic acid’s molecular weight is a crucial parameter that determines its physicochemical and degradable properties. For example, increasing HA’s molecular weight can increase its viscoelasticity.
- Hyaluronic acid has a high affinity to the CD44 receptor, which is commonly over-expressed in cancer cells. This means that hyaluronic acid helps the drug delivery system enter and affect the tumor cells more directly. Hyaluronic acid can bind to these CD44 receptors using six monosaccharide units — HA6.
Despite all of these advantages, nanogels still have yet to be applied to clinical use, meaning that this technology is still nascent and of course, some issues need to be fixed. We will talk about those later. Now that we understand this technologies benefits, and there certainly are a lot of them, let’s talk about how we make this.
Manufacturing
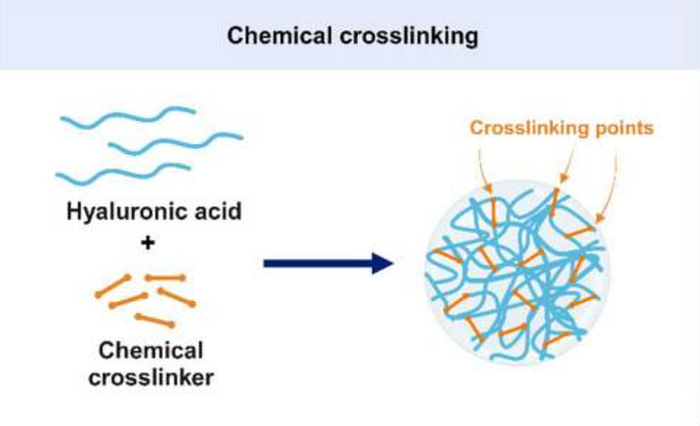
How will this product actually be made? Our nanogel will be manufactured through chemical cross-linking. Nanogels that are formed through chemical crosslinking, which creates strong covalent bonds, are stable in the body. This stability is crucial as it prevents the premature release of drugs by keeping the gel network from falling apart unexpectedly. We will use the most common method: reverse microemulsion crosslinking. In this technique, tiny water-in-oil particles called micelles serve as miniature reactors where hyaluronic acid molecules are crosslinked, allowing for precise control over the size of the resulting nanoparticles. A typical formula for these microemulsions includes water for the hyaluronic acid solution, isooctane as the oil, and sodium bis (ethylhexyl) sulfosuccinate as a surfactant. Finally, 1-heptanol will be added as a co-surfactant to increase microemulsion stability.
For the crosslinking agent, we will use 1,4-butanediol diglycidyl ether (BDDE), which has gained approval from the Food and Drug Administration (FDA). We chose BDDE because it has shown the most significant swelling response to pH changes, making it promising for various biomedical applications where biodegradability and biocompatibility are crucial. Furthermore, the swelling of nanogels in an aqueous environment allows these cargos to be released more efficiently into the surrounding environment.
We will also perform the process of PEGylation, where polyethylene glycol (PEG) chains are conjugated (bonded) to a molecule. PEGylation of the nanogel surface imparts them with ‘stealth’ properties by making the surface more hydrophilic, shielding a charge that the core might carry, and establishing a physical barrier that reduces interactions with blood proteins. However, this is highly dependent on the size of the nanogel, its shape, molecular weight, and surface density of the PEG used. PEGylation is a process in which polyethylene glycol (PEG), a non-toxic and biocompatible polymer, is chemically attached to another molecule, such as a drug or a therapeutic protein. The main purpose of PEGylation is to improve the properties of the molecule it is attached to. This can include increasing the molecule’s stability, solubility, and duration in the body while also reducing immunogenicity (the likelihood of triggering an immune response). By doing so, PEGylation can enhance the efficacy and safety of medications, making them more effective for longer periods and reducing the frequency of dosing.
Injection and Delivery of Our Nanogel
To cross the BBB, our nanogel will first be attached to red blood cells (RBCs) ex vivo and will then use red blood cells to get to the brain in a process known as red blood cell hitchhiking. We choose to attach the nanogel as opposed to loading it inside the RBCs as the nanogel can be released more easily and precisely, making this approach effective and efficient for drug delivery.
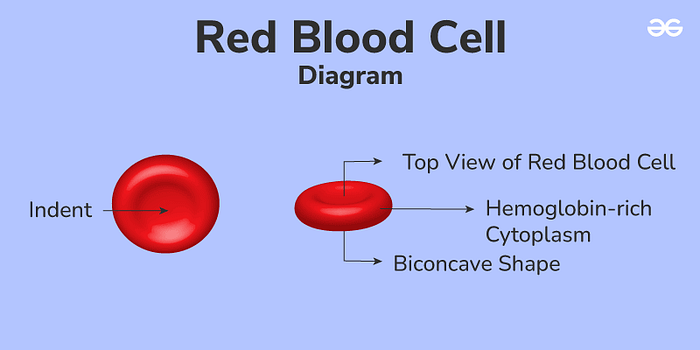
RBCs are abundant, readily available, and relatively expendable. They are simpler and more uniform than other cells, with each cell being about 6–7 micrometers wide and 2 micrometers thick. RBCs are shaped like biconcave disks, which allows them to have a larger surface area and flexibility, allowing them to carry more drugs and have a long-lasting presence in the bloodstream without being detected and eliminated by the immune system. Their incredible durability and flexibility come from their strong, elastic membrane and cytoskeleton, and their lack of nuclei and other internal structures. Not having a nucleus is important, as it provides RBCs with extra room to carry drugs and makes them suitable for genetic modifications. Furthermore, RBCs’ high flexibility allows them to move through very narrow blood vessels, such as those in the spleen and potentially the BBB. Furthermore, since RBCs travel within blood vessels, they minimize the interaction of the encapsulated drugs with other substances, lowering the chance of metabolic breakdown and reducing unintended side effects. Finally, RBCs have proteins on their surface that prevent them from being easily swallowed up by the immune system, allowing them to circulate for long periods and release drugs gradually.
In order to bind the nanogel to the RBC, we will follow a successful process that was outlined in this study. First, we will draw blood from the patient using tubes and then spin the collected blood at a force of 1000 times gravity for 10 minutes at a cold temperature (around 4 degrees Celsius). This process separates the blood into layers, allowing us to remove the top layers containing plasma and the buffy coat, allowing us to reduce the volume of the final unit. We will then wash the remaining RBCs in a saltwater solution, spinning the blood at 500 times gravity for 15 minutes at around 4 degrees Celsius. This washing step allows us to thoroughly clean the RBCs to allow for better binding.
Then we will mix our nanogels with RBCs in a ratio of 200:1. This mixture will be incubated for around an hour under constant rotation again at around 4 degrees Celsius in a phosphate-buffered saline (PBS) solution. After incubation, the nanogel RBC mixture will be washed three times using PBS; the wash involves spinning the mixture at 100 times gravity for 8 minutes to remove any nanogels that had not attached to the RBCs.
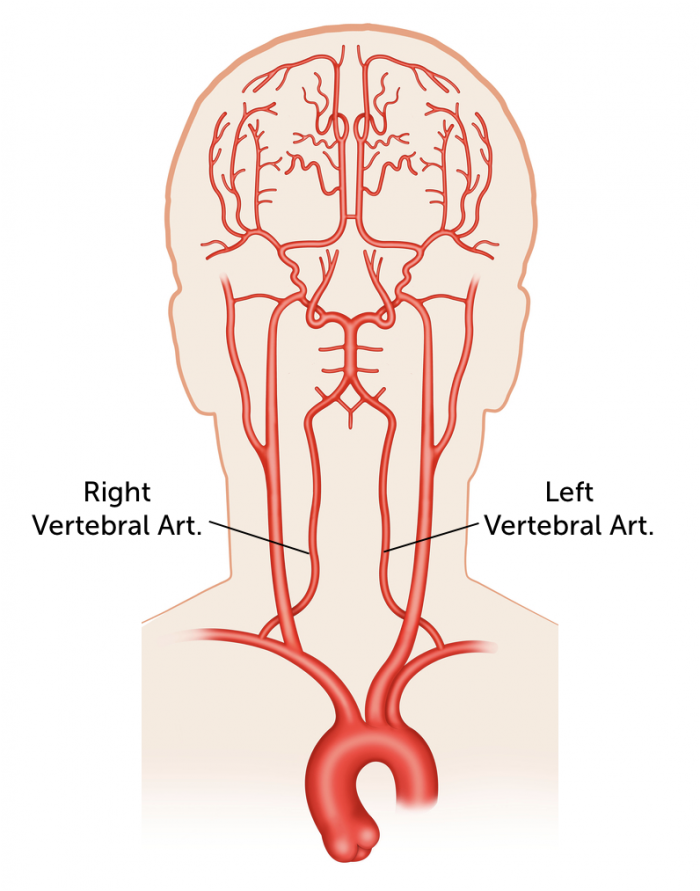
While RBCs normally travel to the lungs, using intra-arterial (IA) catheters, we can direct RBCs loaded with the nanogel to specific organs. Using IA catheters allows the targeting of other organs by injecting the RBC nanogel complex directly into arteries that feed those organs. The arteries that we will target are the vertebral arteries. This is because spinal injections are generally safe procedures. If complications occur, they are usually mild and self-limited. Since blood normally travels to the brain, the RBC will have no problem getting through the BBB and getting to the brain.
Once inside, the nanogel will break apart from the red blood cell and travel to the tumor site. Because hyaluronic acid has a high affinity to cancer cells expressing the CD44 receptor, they have a great desire to find these cells and bind to them. Once it reaches the tumor, these nanogels will be engineered to be sensitive to hypoxic (low oxygen) and highly acidic conditions, which are commonly found within and on the surface of glioblastoma tumors. Thus, when the nanogel encounters this environment it will slowly degrade and slowly release the drug payload. Sustained drug release, slow release of a drug at a programmed rate to deliver the drug over a prolonged period of time, is extremely advantageous, mainly because it reduces the number of doses, lowering expenses and improving patient compliance. Furthermore, sustained drug release also decreases side effects and provides the ability to maintain a constant level of medication within the body. Releasing drugs directly in the tumor (otherwise known as targeted drug delivery) leads to greatly enhanced therapeutic efficacy because the drug is already at the site of action, making it easier to eliminate cancer cells while also not destroying healthy cells. Furthermore, targeted drug delivery can expand the pool of drugs that can be safely and effectively administered, enabling the development of novel therapies for previously untreatable conditions.
We took into account numerous safety considerations when designing this method. For example, that same study showed that attaching nanogels to RBCs would not cause the RBC to clump together. Clumping is dangerous because it can lead to blockages in blood vessels, which could cause a heart attack or stroke. The paper’s results (linked above) showed that nanogels did not cause significant clumping of the RBCs. Furthermore, it was also found that nanogels attached to RBCs did not influence oxygen levels in the blood. Comparing blood oxygen levels between mice injected with nanogels attached to RBCs and plain RBCs, there was no difference in blood oxygen levels.
We have confidence in this approach because it has already been tested on mice and produced extremely promising results. When injecting free nanoparticles (ones that did not bind to RBCs) and nanoparticles bound to RBCs directly into the right internal carotid artery of mice, a major artery that supplies blood to the brain, the study found that the brain-to-blood ratio was 27 times higher with the nanoparticles bound to RBCs than with free nanoparticles, indicating effective localization within the brain rather than remaining in the bloodstream. In assessing any potential damages caused by the red blood cell hitchhiking method, the results showed no morphological differences in the brain, suggesting that the method did not cause acute toxicity in the brain tissue.
Therapeutic
Now you may be wondering what our drug of choice is. We believe that the main issue is getting the drug to the tumor and that targeted drug delivery will by itself greatly enhance therapeutic efficacy. That is why we also emphasized having a flexible system that can adapt based on the newest and most efficient therapies. This drug delivery system can be specifically designed to cater to the specific treatment used and hold multiple treatments at the same time. There is tons of research being done into designing new and more effective therapies for glioblastoma, but not enough about delivering the drug and overcoming the main problem: the BBB. For reference, when you go on Google Scholar, there are over 445 papers on “therapies for glioblastoma” but only around 14 for “drug delivery for glioblastoma.” In fact, here is a study published just last month on a new potential therapy for glioblastoma. At the present moment however, the idea is to do combinatorial therapy, where we would combine a drug such temozolomide, a type of chemotherapy that is already largely used to treat glioblastoma and is considered the “gold standard,” with another promising drug to more effectively treat glioblastoma. For example, we could add treatments such as bevacizumab, which is typically used as a second-line treatment for recurrent glioblastomas.
Challenges
Of course, this solution is not perfect and some hurdles must be overcome. After all, if it was perfect it would have already been done.
On the surface level, an obvious challenge is the FDA and passing clinical trials. As with any implantable system, FDA regulations will be strict and testing will be even more imperative. Furthermore, making sure that this solution can pass all phases of clinical trials (not just the first ones) will be important as if we can’t get our solution to the patient, all of the money spent on research will be pointless.
Furthermore, one of the main challenges in making nanogels through chemical crosslinking is controlling their shape and size. Hyaluronic acid can turn into either a large gel network or tiny particles depending on the technique used. Our chosen method of mixing the hyaluronic acid in a water-in-oil mixture before adding the chemicals is an effective method to control the shape and size of nanogels. In this method, factors such as the ratio of water to oil, the types of surfactants (substances that reduce surface tension) used, and how vigorously the mixture is stirred can control the size of the hyaluronic particles.
Another challenge is the presence of surface charge on a nanogel. Nanogels with nearly neutral surface charge circulate longer in the blood. On the other hand, a key property of nanogels is their ability to respond to environmental changes, which is often enabled by charged groups in their structure. These charged groups also help attach drugs to the nanogel, making it challenging to balance responsive behavior with minimizing unwanted interactions with the body.
Being precise with the amount of crosslinking agent is also critical. If the amount of crosslinking agent used is too small, then the physical interaction between polymer bonds breaks easily, causing the nanogel to become water soluble and could result in it dissolving too early. However, if too much crosslinking agent is used, then a high crosslinking degree causes a low swelling degree of hydrogel, which, as discussed, makes the drug release process a lot harder.
In a study testing chemical crosslinking using BDDE, high crosslinking yields of over 90% were confirmed for all samples except when a 1:10 hyaluronic acid to BDDE molecular weight ratio was used. In these cases, the excess BDDE caused problems due to a large amount of unreacted BDDE, which limits the effectiveness (reduced solubility, improved elasticity, and enhanced mechanical strength and stability) of using high concentrations of BDDE in making hyaluronic acid nanogels. It was also found that using a low amount of BDDE significantly impacted the size of the nanogels, regardless of the HA’s molecular weight. Nanogels made with just 0.2 equivalents of BDDE had much larger particle sizes compared to those made with higher ratios of hyaluronic acid to BDDE (1:1 and 1:10). These low-crosslinked nanogels (1:0.2 hyaluronic acid:BDDE) also displayed better swelling properties, meaning they could absorb more water and expand more when the pH changed or when transitioning from dry to wet states. Thus, we now encounter a situation where we need to evaluate the trade-offs of using a high amount of BDDE versus a low amount.
Consistently reproducing these nanogel-biomolecule combinations is hard due to the unpredictable nature of the chemical reactions used. Additionally, adding targeting molecules to nanogels can change their surface properties, leading to issues like faster clearance from the body.
Furthermore, RBC hitchhiking is a newer method for delivering drugs. As this approach develops and moves towards clinical use, it will encounter various challenges, some of which might be unexpected at this early stage. For instance, in attaching drug-containing nanogels to red blood cells, the nanogel might negatively modify both the drug and the cells. Loading of the drug cargo inevitably alters the RBC, the question is whether changes in RBC biocompatibility are tolerable. For instance, encapsulating the drugs directly into the RBC leads to the loss of hemoglobin, reduced flexibility, and alterations to the cell membrane that could make the RBC more vulnerable to immune system attack. These changes resemble those seen in diseased or aging RBCs, such as those from patients with malaria or sickle cell disease. Moreover, this altered state of RBCs can lead to increased clearance by the immune system’s phagocytes, which poses additional risks including triggering inflammatory responses in the body or even blocking small blood vessels. While these are all issues for encapsulating the drug directly into the RBC, it raises questions of whether similar issues could occur when binding the nanogel to the RBC.
While RBCs are very flexible, they have their limits. If an RBC’s surface area increases by just 3–4%, the cell can burst. This means that careful handling is necessary to maintain the stability of their surface area.
Part II: Poly(lactic-co-glycolic acid) Biosensors
While the hyaluronic acid nanogel is certainly promising (despite the aforementioned challenges), it only works in tandem with the second part of our solution. Ultimately, we still need to solve the tumor recurrence problem and improve cancer imaging as a whole. Thus, we developed an implantable biosensor to provide better imaging of tumors before initial treatment and to spot tumor recurrence early.
As previously discussed, surgery has been crucial for treating solid tumors, but hasn’t changed much over the last century. Surgeons typically use their judgment to feel and see the difference between cancerous and healthy tissues during surgery, which can lead to incomplete removal of the tumor or unnecessary removal of healthy tissue. Furthermore, it is impossible to remove every cancer cell from surgery and surgeons usually can’t risk operating on healthy tissue. There needs be a more precise method to determine how much surgeons can take out and what specific tissues they can operate on. On top of that, after surgery, it’s normal for the brain tissue to shift as it fills the space left by the removed tumor. This can lead to mismatches between the MRI and CT scans used in planning treatment, sometimes with shifts as big as several centimeters. Thus, beyond imaging for tumor recurrence, we need more precise imaging as a whole that provides the doctor with a real-time picture of the tumor and what he is dealing with.
Furthermore, catching tumor recurrence early is essential as it gives us the best shot to stop it before it grows out of control and into healthy brain tissue. Recurrence of glioblastoma is nearly universal, and patients with recurrent glioblastoma fare even worse than the first time, with a median survival of only five to seven months with optimal therapy. Finally, current diagnostic imaging techniques, which include ultrasound, X-ray, CT, MRI, and PET scans, often struggle with low specificity and sensitivity in detecting early-stage cancer. They sometimes do not provide clear differentiation between cancerous, benign, and normal tissues, highlighting the need for more effective imaging methods.
Core Material
We designed a biocompatible and biodegradable system made out of Poly(lactic-co-glycolic acid) (PLGA). PLGA is a copolymer made from two types of acids: poly lactic acid (PLA) and poly glycolic acid (PGA). We choose this material due to its established safety profile and tunable degradation characteristics. Research by Makadia & Siegel demonstrates the biocompatibility and controlled release properties of PLGA nanoparticles, making them ideal for long-term brain applications.
Delivery
Similar to how we delivered our nanogel, we will also use red blood cell hitchhiking to deliver our biosensor across the BBB. Studies by Qie et al., demonstrate the effectiveness of CD47 antibody-conjugated nanoparticles for enhanced RBC adhesions and subsequent BBB penetrations. These antibodies target the CD47 “don’t eat me” signal on RBCs, preventing macrophages from removing them from circulation. Essentially, we will add antibodies that can bind to the CD47 protein on red blood cells.
Sensing the Tumor
In order to detect tumor recurrence, we will focus on the elevated levels of glucose in tumors. Tumors exhibit high glucose levels because cancer cells require a lot of glucose for energy and to produce substances that support their survival, growth, and metastasis. Glucose oxidase (GOx) will be encapsulated within the PLGA core. When GOX encounters glucose, it generates hydrogen peroxide (H202). The presence of H202 then triggers the Cy7 molecule, which can be measured using Fluorescence Resonance Energy Transfer (FRET). Research by Li et al., describes a FRET-based sensor where GOx oxidation of glucose reduces the fluorescence of a reporter molecule, indicating high glucose levels.
Generating a Detectable Signal
Our biosensor will also include a reporter molecule that generates a detectable signal upon interaction with the tumor. Selecting the ideal fluorophore for brain tumor monitoring via red blood cell-hitchhiking biosensors requires careful consideration of several factors:
- Deep tissue penetration. Brain tissue significantly absorbs light, particularly at lower wavelengths. NIR fluorophores are crucial for deeper tissue penetration, allowing for excitation and signal detection through the scalp and skull.
- Excitation and emission wavelengths. The chosen fluorophore should have excitation and emission wavelengths that are distinct from background tissue autofluorescence. Autofluorescence refers to the inherent fluorescence of biological tissues, which can interfere with the biosensor signal.
- Brightness and photostability. High quantum yield (brightness) and photostability (resistance to photobleaching) are essential for strong and sustained signal detection.
For brain tumor monitoring via transcranial fluorescence imaging, near-infrared (NIR) fluorophores are the ideal choice. Here’s why:
- Tissue penetration. Compared to visible light, NIR light experiences less scattering and absorption by biological tissues, allowing for deeper penetration into the brain. This is crucial for reaching brain tumors located deep within the skull.
- Reduced background autofluorescence. Biological tissues exhibit inherent autofluorescence, particularly in the visible spectrum. NIR fluorophores emit at longer wavelengths where background autofluorescence is minimized, offering a clearer signal-to-noise ratio.
In order to select the optimal NIR flurophore, we had to consider various factors:
- Excitation and emission wavelengths. These wavelengths should be chosen to maximize tissue penetration and minimize interference from autofluorescence.
- Quantum Yield (QY). This parameter reflects the efficiency of converting absorbed light into emitted fluorescence. High QY is desirable for a strong signal.
- Photostability. The fluorophore should be resistant to photobleaching, which is the loss of fluorescence intensity upon prolonged exposure to light.
- Biocompatibility. The fluorophore needs to be non-toxic and well-tolerated by the body.
We choose Cy7 for our flurophore, mainly because of its excitation peak at 756 nm and an emission peak at 779 nm, which are both excellent. Similar to our nanogel, Cy7 will undergo the process of PEGylation. This is because PEGylated fluorophores have demonstrated enhanced imaging capabilities, such as better spatial resolution in the vascular and lymphatic systems and significantly higher tumor-to-background signal ratios when tagged with a targeting ligand, compared to similar NIR fluorophores. Moreover, LUM015, a PEGylated activatable fluorophore, is currently in clinical trials for detecting soft-tissue sarcoma and breast cancer, showcasing how PEGylation can effectively improve the usability and efficacy of these imaging agents in clinical settings. This technique not only addresses the solubility and clearance issues of the fluorophores but also helps maintain their safety profile for use in humans.
Sending the Signal
Cy7 will be incorporated within the PLGA core of the biosensor. Once injected, the biosensor reaches the brain and interacts with the target analyte (e.g., tumor marker). This interaction triggers a change within the biosensor, which can be detected by the fluorophore in several ways:
- Fluorescence intensity change. In some cases, the interaction with the target molecule may directly alter the fluorophore’s brightness. For instance, some fluorophores exhibit aggregation-induced quenching (ACQ), where aggregation reduces fluorescence. The biosensor design could leverage ACQ, where upon target binding, fluorophore aggregation leads to a decrease in overall fluorescence intensity.
- Fluorescence Resonance Energy Transfer (FRET). This technique utilizes a donor and acceptor fluorophore pair. The donor fluorophore, upon excitation, transfers its energy to the acceptor fluorophore, resulting in emission at the acceptor’s wavelength. The biosensor can be designed with the target analyte affecting the distance or interaction between the donor and acceptor fluorophores. Changes in this distance will alter FRET efficiency, impacting the detected fluorescence intensity or emission spectrum.
Cost
No matter how innovative or effective our solution is, cost is a key consideration that we must keep in mind. After all, if no one can afford our therapy then all of this work is useless. Our goal was to ensure that this solution cost less than the current treatment regime. Thankfully, this very well could be a reality
Currently, for our two core materials, as mentioned before, the average retail price of hyaluronic acid is typically around $59.69 per 3, 30 capsules bottle. The PLGA copolymer costs $65 for 500mg and $110 for 1g.
However, where the real price considerations come into play is with the fabrication, for both the nanogel and biosensor. While it is currently hard to estimate how much reverse microemulsion crosslinking will cost. We can at least estimate how much it would cost to obtain all the materials necessary. Isooctane, which is our oil, costs $91 dollars for 500mL and $140 for 1L. Sodium bis (ethylhexyl) sulfosuccinate, which functions as our surfactant, costs $415 for 10mg and $1,660 for 100mg Finally, 1-heptanol, our co-surfactant, costs $40 for 500mL and $108 for 2500mL. While these materials are certainly not cheap, the total price to obtain all of these materials and the hyaluronic acid is around $2,000 (assuming we took the larger amount that I noted).
Now for the biosensors, the cost of Cy7 is $180 for 5mg solid and $270 for 1mL aqueous. The two other important costs that I simply can’t find data for would be the cost of the system (FRET or Fluorescence intensity change) that can receive the Cy7 signal and the cost of PEGylation of Cy7.
Finally, we must also consider the costs of synthesizing all of these materials, as the material cost alone is not nearly indicative of the final cost. Some equipment that will definitely be used for the fabrication of both of these systems, especially the nanogels, will be an electrospinning machine, a plasma treatment machine, an incubator, and a centrifuge. Furthermore, everything will be prepared in a sterile cell culture hood. While I don’t know the exact prices, these machines are definitely expensive. Although it is irrational to accept a huge price decrease as the years pass, it is reasonable to assume that the general price to produce nanogels and biosensors will decrease as our technology improves, making their creation even more economical.
Conclusion
Now, with this technology, imagine a world where a glioblastoma diagnosis is not the end of the world. One trip to the doctor and a couple of hours is all it takes to rid you of this disease. Imagine a world where tumor recurrence is not an issue, where there is no need for for constant checkups or expensive routine MRI scans. You have one system, a biosensor, that can alert you immediately when tumor recurrence is spotted. If tumor recurrence occurs, it will be spotted early and taken care of swiftly.
So what are you waiting for! Join our team at Sanavo and lets work to make this imagined future a reality — where having glioblastoma is no longer a death sentence.
If you liked this article, have any questions, or want to provide any feedback, please email me (natank929@gmail.com) or check out my LinkedIn to connect further.
Sources
This article took an insane amount of research and I don’t want to play my ideas off as purely my own. Here is where I got my information from:
- https://www.tandfonline.com/doi/full/10.1080/13696998.2017.1364258
- https://www.americanlifefund.com/cancer/treatment/costs/brain/#:~:text=Brain Surgery Phase Costs Estimates&text=The costs associated with this,anywhere from %2430%2C000 to %24100%2C000.
- https://www.mayoclinic.org/diseases-conditions/glioblastoma/cdc-20350148#dialogId50748516
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10610124/#:~:text=Hydrogels based on natural polymers,which endow them with widespread
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348459/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10610124/#:~:text=Hydrogels based on natural polymers,which endow them with widespread
- https://pubs.rsc.org/en/content/articlelanding/2015/cs/c4cs00341a
- https://pharmacy.unc.edu/2016/06/removing-brain-tumor-makes-remaining-cancer-aggressive-unc-study-finds/
- https://www.cancerresearchuk.org/about-cancer/brain-tumours/treatment/surgery/recovering
- https://www.thebraintumourcharity.org/brain-tumour-diagnosis-treatment/treating-brain-tumours/adult-treatments/chemotherapy/temozolomide/
- https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Glioblastoma-Multiforme
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8246629/#:~:text=The BBB is disrupted during,in brain tumours3–6.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9144811/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8277719/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5424548/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8162335/
- https://news.mit.edu/2019/how-tumors-behave-acid-0320#:~:text=Scientists have long known that,become more invasive and metastatic.
- https://www.mskcc.org/cancer-care/patient-education/about-your-ommaya-reservoir-placement
- https://www.medicalnewstoday.com/articles/323482#cost
- https://www.cancerresearchuk.org/about-cancer/brain-tumours/treatment/chemotherapy-treatment#:~:text=Chemotherapy uses anti cancer
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8835860/#:~:text=One such biodegradable polymer delivery,for treating GBM [11].
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9498307/#:~:text=If the amount of crosslinking,low swelling degree of hydrogel.
- https://www.nature.com/articles/s41467-018-05079-7#Sec11
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5683405/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4422082/#:~:text=CD44%20Binds%20to%20Soluble%20Extracellular%20HA%20Molecules%20and%20ECM&text=CD44%20is%20endogenously%20expressed%20at,six%20monosaccharide%20units%20(HA6).
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8156977/
